Ununpentium
About this schools Wikipedia selection
This wikipedia selection has been chosen by volunteers helping SOS Children from Wikipedia for this Wikipedia Selection for schools. Before you decide about sponsoring a child, why not learn about different sponsorship charities first?
| Ununpentium | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
115Uup
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||
| unknown | |||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||
| Name, symbol, number | ununpentium, Uup, 115 | ||||||||||||||||||||||||||||||
| Pronunciation | / uː n uː n ˈ p ɛ n t i ə m / oon-oon-PEN-tee-əm |
||||||||||||||||||||||||||||||
| Metallic category | unknown | ||||||||||||||||||||||||||||||
| Group, period, block | 15 (pnictogens), 7, p | ||||||||||||||||||||||||||||||
| Standard atomic weight | [288] | ||||||||||||||||||||||||||||||
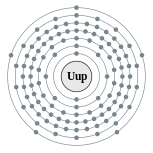
| Electron configuration | [Rn] 5f14 6d10 7s2 7p3 (predicted) 2, 8, 18, 32, 32, 18, 5 (predicted) |
||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||
| Discovery | Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2003) | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | solid (predicted) | ||||||||||||||||||||||||||||||
| Density (near r.t.) | 11 (predicted) g·cm−3 | ||||||||||||||||||||||||||||||
| Melting point | ~700 K, ~430 °C, ~810 (predicted) °F | ||||||||||||||||||||||||||||||
| Boiling point | ~1400 K, ~1100 °C, ~2000 (predicted) °F | ||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||
| Oxidation states | 1, 3 (prediction) | ||||||||||||||||||||||||||||||
| Ionization energies | 1st: 538.4 (prediction) kJ·mol−1 | ||||||||||||||||||||||||||||||
| Atomic radius | 200 (predicted) pm | ||||||||||||||||||||||||||||||
| Covalent radius | 162 (estimated) pm | ||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||
| CAS registry number | 54085-64-2 | ||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||
| Main article: Isotopes of ununpentium | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
 |
Ununpentium
Common English pronunciation of ununpentium
|
| Problems listening to this file? See media help. | |
Ununpentium is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115.
It is placed as the heaviest member of group 15 (VA) although a sufficiently stable isotope is not known at this time that would allow chemical experiments to confirm its position as a heavier homologue to bismuth. It was first observed in 2003 and about 50 atoms of ununpentium have been synthesized to date, with about 25 direct decays of the parent element having been detected. Four consecutive isotopes are currently known, 287–290Uup, with 289Uup having the longest measured half-life of ~200 ms.
History
Discovery profile
On February 2, 2004, synthesis of ununpentium was reported in Physical Review C by a team composed of Russian scientists at the Joint Institute for Nuclear Research in Dubna, and American scientists at the Lawrence Livermore National Laboratory. The team reported that they bombarded americium-243 with calcium-48 ions to produce four atoms of ununpentium. These atoms, they report, decayed by emission of alpha-particles to ununtrium in approximately 100 milliseconds.
- 48
20Ca + 243
95Am → 291
115Uup*
→ 288
115Uup + 3 n → 284
113Uut + α
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununpentium by conducting chemical experiments on the decay daughter 268Db. In experiments in June 2004 and December 2005, the dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities. Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 to the parent nuclei.
Sergei Dmitriev from the Flerov Laboratory of Nuclear Reactions (FLNR) in Dubna, Russia, has formally put forward their claim of discovery of ununpentium to the IUPAC/IUPAP Joint Working Party (JWP). In 2011, the IUPAC evaluated the Dubna-Livermore results and concluded that they did not meet the criteria for discovery.
Naming
Ununpentium is historically known as eka-bismuth. Ununpentium is a temporary IUPAC systematic element name derived from the digits 115, where "un-" represents Latin unum. "Pent-" represents the Greek word for 5, and it was chosen because the Latin word for 5 ("quin") starts with 'q', which would have caused confusion with flerovium (previously known as ununquadium), element 114. Research scientists usually refer to the element simply as element 115.
Current and future experiments
The team at Dubna are currently running another series of experiments on the 243Am(48Ca,xn) reaction. They are attempting to complete the 4n excitation function and confirm the data for 287115. They are also hoping to identify some decays from the 2n and 5n exit channels. This reaction will run until the Christmas shutdown.
The FLNR also have future plans to study light isotopes of element 115 using the reaction 241Am + 48Ca.
Nucleosynthesis
- Target-projectile combinations leading to Z=115 compound nuclei
The table below contains various combinations of targets and projectiles which could be used to form compound nuclei with Z=115. The table below contains various target-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 75As | 283Uup | Reaction yet to be attempted |
| 232Th | 55Mn | 287Uup | Reaction yet to be attempted |
| 238U | 51V | 289Uup | Failure to date |
| 237Np | 50Ti | 287Uup | Reaction yet to be attempted |
| 244Pu | 45Sc | 289Uup | Reaction yet to be attempted |
| 243Am | 48Ca | 291Uup | Successful reaction |
| 241Am | 48Ca | 289Uup | Planned Reaction |
| 248Cm | 41K | 289Uup | Reaction yet to be attempted |
| 249Bk | 40Ar | 289Uup | Reaction yet to be attempted |
| 249Cf | 37Cl | 286Uup | Reaction yet to be attempted |
Hot fusion
This section deals with the synthesis of nuclei of ununpentium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40–50 MeV, hence "hot"), leading to a reduced probability of survival from fission. The excited nucleus then decays to the ground state via the emission of 3–5 neutrons. Fusion reactions utilizing 48Ca nuclei usually produce compound nuclei with intermediate excitation energies (~30–35 MeV) and are sometimes referred to as "warm" fusion reactions. This leads, in part, to relatively high yields from these reactions.
- 238U(51V,xn)289−xUup
There are strong indications that this reaction was performed in late 2004 as part of a uranium(IV) fluoride target test at the GSI. No reports have been published suggesting that no products atoms were detected, as anticipated by the team.
- 243Am(48Ca,xn)291−xUup (x=2,3,4)
This reaction was first performed by the team in Dubna in July–August 2003. In two separate runs they were able to detect 3 atoms of 288Uup and a single atom of 287Uup. The reaction was studied further in June 2004 in an attempt to isolate the descendant 268Db from the 288Uup decay chain. After chemical separation of a +4/+5 fraction, 15 SF decays were measured with a lifetime consistent with 268Db. In order to prove that the decays were from dubnium-268, the team repeated the reaction in August 2005 and separated the +4 and +5 fractions and further separated the +5 fractions into tantalum-like and niobium-like ones. Five SF activities were observed, all occurring in the +5 fractions and none in the tantalum-like fractions, proving that the product was indeed isotopes of dubnium.
In a series of experiments between October 2010 - February 2011, scientists at the FLNR studied this reaction at a range of excitation energies. They were able to detect 21 atoms of 288115 and one atom of 289115, from the 2n exit channel. This latter result was used to support the synthesis of ununseptium. The 3n excitation function was completed with a maximum at ~8 pb. The data was consistent with that found in the first experiments in 2003.
Isotopes and nuclear properties
- Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 287Uup | 2003 | 243Am(48Ca,4n) |
| 288Uup | 2003 | 243Am(48Ca,3n) |
| 289Uup | 2009 | 249Bk(48Ca,4n) |
| 290Uup | 2009 | 249Bk(48Ca,3n) |
Theoretical calculations using a quantum-tunneling model support the experimental alpha-decay half-lives.
Chemical properties
Extrapolated chemical properties
Oxidation states
Ununpentium is projected to be the third member of the 7p series of chemical elements and the heaviest member of group 15 (VA) in the Periodic Table, below bismuth. In this group, each member is known to portray the group oxidation state of +V but with differing stability. For nitrogen, the +V state is very difficult to achieve due to the lack of low-lying d- orbitals and the inability of the small nitrogen atom to accommodate five ligands. The +V state is well represented for phosphorus, arsenic, and antimony. However, for bismuth it is rare due to the reluctance of the 6s2 electrons to participate in bonding. This effect is known as the "inert pair effect" and is commonly linked to relativistic stabilisation of the 6s-orbitals. It is expected that ununpentium will continue this trend and portray only +III and +I oxidation states. Nitrogen(I) and bismuth(I) are known but rare and ununpentium(I) is likely to show some unique properties. Because of spin-orbit coupling, flerovium may display closed-shell or noble gas-like properties; if this is the case, ununpentium will likely be monovalent as a result, since the cation Uup+ will have the same electron configuration as flerovium.
Chemistry
Ununpentium should display eka-bismuth chemical properties and should therefore form a sesquioxide, Uup2O3, and analogous chalcogenides, Uup2S3, Uup2Se3 and Uup2Te3. It should also form tri hydrides and tri halides, i.e. UupH3, UupF3, UupCl3, UupBr3 and UupI3. If the +V state is accessible, it is likely that it is only possible in the fluoride, UupF5.
Stability
All the reported above isotopes of element 115, obtained by nuclear collisions of lighter nuclei, are severely neutron-deficient, because the proportion of neutrons to protons needed for maximum stability increases with atomic number. The most stable isotope will probably be 299Uup, with 184 neutrons, a known "magic" closed-shell number conferring exceptional stability, making it (with one further proton outside the "magic number" of 114 protons) both the chemical and the nuclear homolog of 209Bi; but the technology required to add the required neutrons presently does not exist. This is because no known combination of target and projectile can result in the required neutrons. It has been suggested that such a neutron-rich isotope could be formed by quasifission (fusion followed by fission) of a massive nucleus, multi-nucleon transfer reactions in collisions of actinide nuclei, or by the alpha decay of a massive nucleus (although this would depend on the stability of the parent nuclei towards spontaneous fission). One way to create 299Uup would be:
193
77Ir(132
50Sn, 2n) → 323
127Ubs → 299
115Uup + 6 α