Argon
About this schools Wikipedia selection
This Schools selection was originally chosen by SOS Children for schools in the developing world without internet access. It is available as a intranet download. Click here to find out about child sponsorship.
| Argon | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
18Ar
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||||||||
colorless gas exhibiting a lilac/violet glow when placed in a high voltage electric field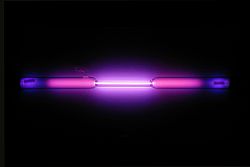 Spectral lines of argon |
|||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | argon, Ar, 18 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | / ˈ ɑr ɡ ɒ n / | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metallic category | noble gases | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 18 (noble gases), 3, p | ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 39.948(1) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p6 2, 8, 8 |
||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Lord Rayleigh and William Ramsay (1894) | ||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Lord Rayleigh and William Ramsay (1894) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | gas | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 1.784 g/L |
||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at b.p. | 1.40 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 83.80 K, −189.35 °C, −308.83 °F | ||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 87.30 K, −185.85 °C, −302.53 °F | ||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 83.8058 K (-189°C), 69 kPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 150.87 K, 4.898 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 1.18 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 6.43 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 5 R/2 = 20.786 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | no data (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 1520.6 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 2665.8 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3931 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 106±10 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 188 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic | ||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 17.72x10-3 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | (gas, 27 °C) 323 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440–37–1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of argon | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Argon is a chemical element with symbol Ar and atomic number 18. It is in group 18 (noble gases) of the periodic table. Argon is the third most common gas in the Earth's atmosphere, at 0.93% (9,300 ppm), making it approximately 23.8 times as abundant as next most common atmospheric gas, carbon dioxide (390 ppm), and more than 500 times as abundant as the next most common noble gas, neon (18 ppm). Nearly all of this argon is radiogenic argon-40 derived from the decay of potassium-40 in the Earth's crust. In the universe, argon-36 is by far the most common argon isotope, being the preferred argon isotope produced by stellar nucleosynthesis in supernovas.
The name "argon" is derived from the Greek word αργον meaning "lazy" or "the inactive one", a reference to the fact that the element undergoes almost no chemical reactions. The complete octet (eight electrons) in the outer atomic shell makes argon stable and resistant to bonding with other elements. Its triple point temperature of 83.8058 K is a defining fixed point in the International Temperature Scale of 1990.
Argon is produced industrially by the fractional distillation of liquid air. Argon is mostly used as an inert shielding gas in welding and other high-temperature industrial processes where ordinarily non-reactive substances become reactive; for example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning. Argon gas also has uses in incandescent and fluorescent lighting, and other types of gas discharge tubes. Argon makes a distinctive blue-green gas laser.
Characteristics
Argon has approximately the same solubility in water as oxygen, and is 2.5 times more soluble in water than nitrogen. Argon is colorless, odorless, and nontoxic as a solid, liquid, and gas. Argon is chemically inert under most conditions and forms no confirmed stable compounds at room temperature.
Although argon is a noble gas, it has been found to have the capability of forming some compounds. For example, the creation of argon fluorohydride (HArF), a marginally stable compound of argon with fluorine and hydrogen, was reported by researchers at the University of Helsinki in 2000. Although the neutral ground-state chemical compounds of argon are presently limited to HArF, argon can form clathrates with water when atoms of it are trapped in a lattice of the water molecules. Argon-containing ions and excited state complexes, such as ArH+ and ArF, respectively, are known to exist. Theoretical calculations have predicted several argon compounds that should be stable, but for which no synthesis routes are currently known.
History

Argon (αργος, Greek meaning "inactive", in reference to its chemical inactivity) was suspected to be present in air by Henry Cavendish in 1785 but was not isolated until 1894 by Lord Rayleigh and Sir William Ramsay in Scotland in an experiment in which they removed all of the oxygen, carbon dioxide, water and nitrogen from a sample of clean air. They had determined that nitrogen produced from chemical compounds was one-half percent lighter than nitrogen from the atmosphere. The difference seemed insignificant, but it was important enough to attract their attention for many months. They concluded that there was another gas in the air mixed in with the nitrogen. Argon was also encountered in 1882 through independent research of H. F. Newall and W.N. Hartley. Each observed new lines in the colour spectrum of air but were unable to identify the element responsible for the lines. Argon became the first member of the noble gases to be discovered. The symbol for argon is now Ar, but up until 1957 it was A.
Occurrence
Argon constitutes 0.934% by volume and 1.28% by mass of the Earth's atmosphere, and air is the primary raw material used by industry to produce purified argon products. Argon is isolated from air by fractionation, most commonly by cryogenic fractional distillation, a process that also produces purified nitrogen, oxygen, neon, krypton and xenon.
Isotopes
The main isotopes of argon found on Earth are 40Ar (99.6%), 36Ar (0.34%), and 38Ar (0.06%). Naturally occurring 40K with a half-life of 1.25×109 years, decays to stable 40Ar (11.2%) by electron capture or positron emission, and also to stable 40Ca (88.8%) via beta decay. These properties and ratios are used to determine the age of rocks by the method of K-Ar dating.
In the Earth's atmosphere, 39Ar is made by cosmic ray activity, primarily with 40Ar. In the subsurface environment, it is also produced through neutron capture by 39K or alpha emission by calcium. 37Ar is created from the neutron spallation of 40Ca as a result of subsurface nuclear explosions. It has a half-life of 35 days.
Argon is notable in that its isotopic composition varies greatly between different locations in the solar system. Where the major source of argon is the decay of 40K in rocks, 40Ar will be the dominant isotope, as it is on Earth. Argon produced directly by stellar nucleosynthesis, in contrast, is dominated by the alpha process nuclide, 36Ar. Correspondingly, solar argon contains 84.6% 36Ar based on solar wind measurements.
The predominance of radiogenic 40Ar is responsible for the fact that the standard atomic weight of terrestrial argon is greater than that of the next element, potassium. This was puzzling at the time when argon was discovered, since Mendeleev had placed the elements in his periodic table in order of atomic weight, although the inertness of argon implies that it must be placed before the reactive alkali metal potassium. Henry Moseley later solved this problem by showing that the periodic table is actually arranged in order of atomic number. (See History of the periodic table).
The much greater atmospheric abundance of argon relative to the other noble gases is also due to the presence of radiogenic 40Ar. Primordial 36Ar has an abundance of only 31.5 ppmv (= 9340 ppmv x 0.337%), comparable to that of neon (18.18 ppmv).
The Martian atmosphere contains 1.6% of 40Ar and 5 ppm of 36Ar. The Mariner space probe fly-by of the planet Mercury in 1973 found that Mercury has a very thin atmosphere with 70% argon, believed to result from releases of the gas as a decay product from radioactive materials on the planet. In 2005, the Huygens probe also discovered the presence of 40Ar on Titan, the largest moon of Saturn.
Compounds
Argon's complete octet of electrons indicates full s and p subshells. This full outer energy level makes argon very stable and extremely resistant to bonding with other elements. Before 1962, argon and the other noble gases were considered to be chemically inert and unable to form compounds; however, compounds of the heavier noble gases have since been synthesized. In August 2000, the first argon compound was formed by researchers at the University of Helsinki. By shining ultraviolet light onto frozen argon containing a small amount of hydrogen fluoride with caesium iodide, argon fluorohydride (HArF) was formed. It is stable up to 40 kelvin (−233 °C). The metastable ArCF2+
2 dication, which is valence isoelectronic with carbonyl fluoride, was observed in 2010.
Production
Industrial
Argon is produced industrially by the fractional distillation of liquid air in a cryogenic air separation unit; a process that separates liquid nitrogen, which boils at 77.3 K, from argon, which boils at 87.3 K, and liquid oxygen, which boils at 90.2 K. About 700,000 tonnes of argon are produced worldwide every year.
In radioactive decays
40Ar, the most abundant isotope of argon, is produced by the decay of 40K with a half-life of 1.25×109 years by electron capture or positron emission. Because of this, it is used in potassium-argon dating to determine the age of rocks.
Applications

There are several different reasons argon is used in particular applications:
- An inert gas is needed. In particular, argon is the cheapest alternative when nitrogen is not sufficiently inert.
- Low thermal conductivity is required.
- The electronic properties (ionization and/or the emission spectrum) are necessary.
Other noble gases would probably work as well in most of these applications, but argon is by far the cheapest. Argon is inexpensive since it is a byproduct of the production of liquid oxygen and liquid nitrogen from a cryogenic air separation unit, both of which are used on a large industrial scale. The other noble gases (except helium) are produced this way as well, but argon is the most plentiful by far, since it has a much higher concentration in the atmosphere. The bulk of argon applications arise simply because it is inert and relatively cheap.
Industrial processes
Argon is used in some high-temperature industrial processes, where ordinarily non-reactive substances become reactive. For example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning.
For some of these processes, the presence of nitrogen or oxygen gases might cause defects within the material. Argon is used in various types of arc welding such as gas metal arc welding and gas tungsten arc welding, as well as in the processing of titanium and other reactive elements. An argon atmosphere is also used for growing crystals of silicon and germanium.
Argon is an asphyxiant in the poultry industry, either for mass culling following disease outbreaks, or as a means of slaughter more humane than the electric bath. Argon's relatively high density causes it to remain close to the ground during gassing. Its non-reactive nature makes it suitable in a food product, and since it replaces oxygen within the dead bird, argon also enhances shelf life.
Argon is sometimes used for extinguishing fires where damage to equipment is to be avoided.
Scientific research
Argon is used, primarily in liquid form, as the target for direct dark matter searches. The interaction of a hypothetical WIMP particle with the argon nucleus produces scintillation light that is then detected by photomultiplier tubes. Two-phase detectors also use argon gas to detect the ionized electrons produced during the WIMP-nucleus scattering. As with most other liquefied noble gases, argon has a high scintillation lightyield (~ 51 photons / keV), is transparent to its own scintillation light, and is relatively easy to purify. Compared to xenon, argon is cheaper and has a distinct scintillation time profile which allows the separation of electronic recoils from nuclear recoils. On the other hand, its intrinsic gamma-ray background is larger due to 39Ar contamination, unless one uses underground argon sources with a low level of radioactivity. Dark matter detectors currently operating with liquid argon include WArP, ArDM, microCLEAN and DEAP-I.
Preservative

Argon is used to displace oxygen- and moisture-containing air in packaging material to extend the shelf-lives of the contents (argon has the European food additive code of E938). Aerial oxidation, hydrolysis, and other chemical reactions which degrade the products are retarded or prevented entirely. Bottles of high-purity chemicals and certain pharmaceutical products are available in sealed bottles or ampoules packed in argon. In wine making, argon is used to top-off barrels to avoid the aerial oxidation of ethanol to acetic acid during the aging process.
Argon is also available in aerosol-type cans, which may be used to preserve compounds such as varnish, polyurethane, paint, etc. for storage after opening.
Since 2002, the American National Archives stores important national documents such as the Declaration of Independence and the Constitution within argon-filled cases to retard their degradation. Using argon reduces gas leakage, compared with the helium used in the preceding five decades.
Laboratory equipment
Argon may be used as the inert gas within Schlenk lines and gloveboxes. The use of argon over comparatively less expensive nitrogen is preferred where nitrogen may react with the experimental reagents or apparatus.
Argon may be used as the carrier gas in gas chromatography and in electrospray ionization mass spectrometry; it is the gas of choice for the plasma used in ICP spectroscopy. Argon is preferred for the sputter coating of specimens for scanning electron microscopy. Argon gas is also commonly used for sputter deposition of thin films as in microelectronics and for wafer cleaning in microfabrication.
Medical use
Cryosurgery procedures such as cryoablation use liquefied argon to destroy cancer cells. In surgery it is used in a procedure called "argon enhanced coagulation" which is a form of argon plasma beam electrosurgery. The procedure carries a risk of producing gas embolism in the patient and has resulted in the death of one person via this type of accident. Blue argon lasers are used in surgery to weld arteries, destroy tumors, and to correct eye defects. It has also been used experimentally to replace nitrogen in the breathing or decompression mix, to speed the elimination of dissolved nitrogen from the blood. See Argox.
Lighting

Incandescent lights are filled with argon, to preserve the filaments at high temperature from oxidation. It is used for the specific way it ionizes and emits light, such as in plasma globes and calorimetry in experimental particle physics. Gas-discharge lamps filled with argon provide blue light. Argon is also used for the creation of blue and green laser light.
Safety
Although argon is non-toxic, it is 38% denser than air and is therefore considered a dangerous asphyxiant in closed areas. It is also difficult to detect because it is colorless, odorless, and tasteless. A 1994 incident in which a man was asphyxiated after entering an argon filled section of oil pipe under construction in Alaska highlights the dangers of argon tank leakage in confined spaces, and emphasizes the need for proper use, storage and handling.