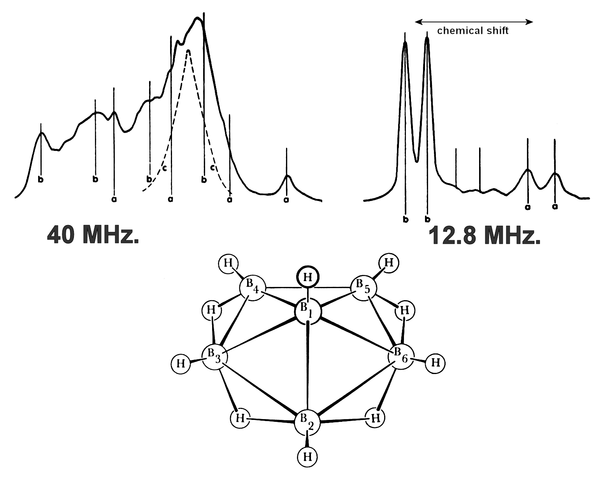
English: Nuclear Magnetic Resonance NMR spectrum and structure of hexaborane B6H10.NMR Interpretation of hexaborane
12.8 MHz. range (right)
A simple example of the chemical shift is the "a" and "b" doublets (pairs of peaks). "A" and "b" are two groups of boron atoms in different electronic environments, which place the "a" doublet to the right of the "b" doublet in the spectrum.
The relative peak heights of the "a" and "b" doublets suggest a 1:5 ratio of borons in one environment to borons in another environment.
This suggests, combined with the molecular weight of the molecule:
- 5 boron atoms in one environment ("b") and
- 1 boron atom in another environment ("a"),
which in turn suggests a pyramidal structure, which does appear in the atomic diagram.
40 MHz. range (left)
"a" suggests a hydrogen at the apex, which is in the atomic diagram sticking straight up.
"b" suggests hydrogens bonded to one boron each, which are in the atomic diagram sticking straight out from the edges.
"c" suggests other bridge hydrogens, which is to say hydrogens in the middle of a boron-hydrogen-boron bonding arrangement like a hydrogen bridge between two boron shores, which are in a ring around the atomic diagram.
(Williams1959)
History
X-ray diffraction was needed to be sure of the structure.(Hirshfeld1958)
More complex molecules are more challenging, although in some cases common subgroups of atoms produce characteristic spectral patterns, which can be recognized.
References
(Williams1959) Wiliams, R.E., Gibbins, S.G., and Shapiro, I., J. Chem. Phys, 30, 333 (1959).
(Hirshfeld1958) Hirshfeld, F. L., Eriks, K., Dickerson, R. E., Lippert, E. L., and Lipscomb, W. N., "Molecular and Crystal Structure of B
6H
10,”
J. Chem. Phys. 28, 56 (1958).