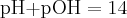Formula Sheet
Gases, Liquids, and Solutions
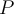 = pressure,
= pressure, 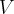 = volume,
= volume, 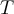 = temperature,
= temperature, 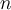 = number of moles,
= number of moles, 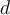 = density,
= density, 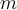 = mass,
= mass, 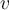 = velocity,
= velocity, 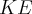 = kinetic energy,
= kinetic energy, 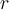 = rate of effusion,
= rate of effusion, 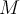 = molar mass,
= molar mass, 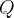 = reaction quotient,
= reaction quotient, 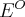 = standard reduction potential,
= standard reduction potential, 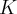 = equilibrium constant
= equilibrium constant
Atomic Structure
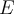 = energy,
= energy, 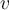 = frequency or
= frequency or 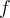 = frequency,
= frequency, 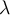 = wavelength,
= wavelength,  = velocity,
= velocity, 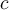 = speed of light =
= speed of light = 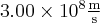
Equilibrium
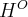 = standard enthalpy,
= standard enthalpy, 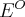 = standard reduction potential,
= standard reduction potential, 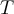 = temperature,
= temperature, 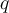 = heat,
= heat, 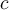 = specific heat capacity,
= specific heat capacity, 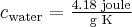 ,
, 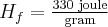 for water,
for water, 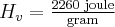 for water
for water
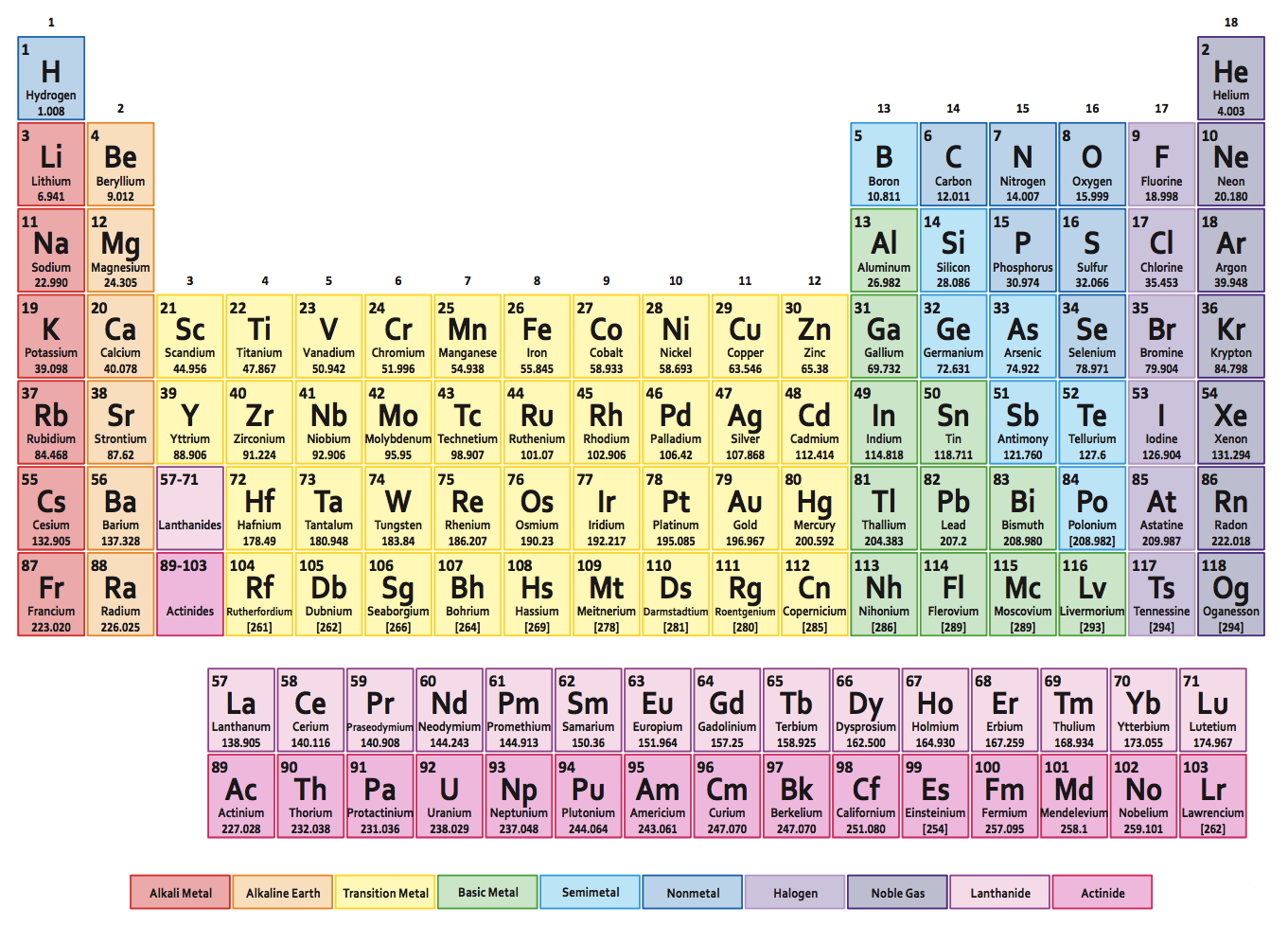
Periodic Table of the Elements
Last modified: Monday, June 26, 2017, 12:40 PM
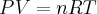
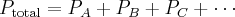
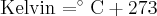
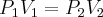
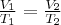
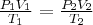
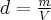
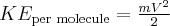
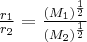
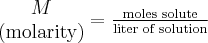
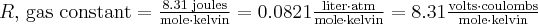
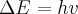
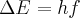
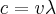
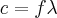
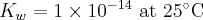
![\textup{pH}=-\textup{log}\left [ \textup{H}^+ \right ], \textup{ pOH}=-\textup{log}\left [ \textup{OH}^- \right ] \textup{pH}=-\textup{log}\left [ \textup{H}^+ \right ], \textup{ pOH}=-\textup{log}\left [ \textup{OH}^- \right ]](../../filter/tex/pix.php/06c7ff5a35da804d2be20d7ad51babf2.png)