Concept
Version 8
Created by Boundless
Speed Distribution of Molecules
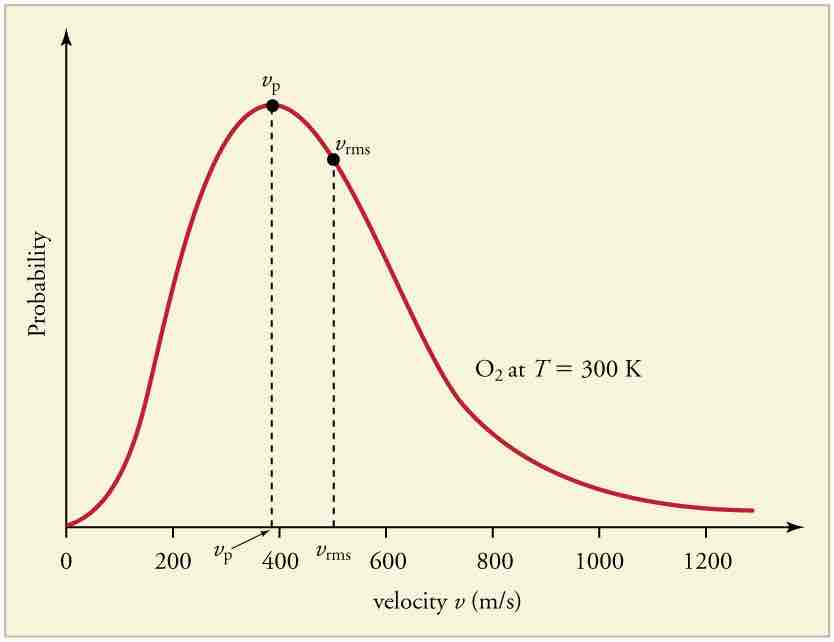
Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution of molecular speeds in an ideal gas. The most likely speed v_p is less than the rms speed v_rms. Although very high speeds are possible, only a tiny fraction of the molecules have speeds that are an order of magnitude greater than v_rms.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature. February 4, 2013."
http://cnx.org/content/m42217/latest/
OpenStax CNX
CC BY 3.0.