Section 2
Bohr's Theory
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
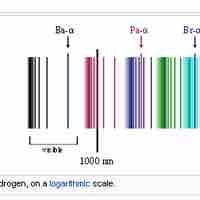
Emission Spectrum of the Hydrogen Atom
The emission spectrum of atomic hydrogen is divided into a number of spectral series.
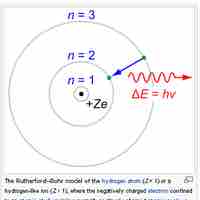
The Bohr Model
The Bohr model depicts atoms as small, positively charged nuclei surrounded by electrons in circular orbits.
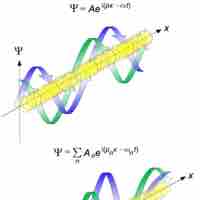
The de Broglie Wavelength
The de Broglie wavelength is inversely proportional to the momentum of a particle.
The Uncertainty Principle
Only partial knowledge of the momentum and position of a particle may be known at the same time.