Chapter 14
Chemical Equilibrium
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
Section 2
Writing Equilibrium Constant Expressions
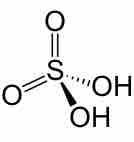
Heterogeneous and Multiple Equilibria
Heterogeneous equilibria involve reactions with compounds in different phases; multiple equilibria involve reactions with two or more steps.
Specialized Equilibrium Constants
Common reactions, such as the self-ionization of water, have specially named equilibrium constants.
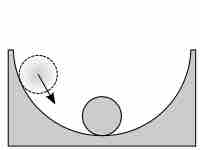
Reaction Quotients
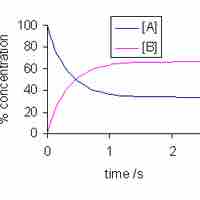
The reaction quotient is a measure of the relative amounts of reactants and products during a chemical reaction at a given point in time.

Expressing the Equilibrium Constant of a Gas in Terms of Pressure
For gas-phase reactions, the equilibrium constant can be expressed in terms of partial pressures, and is given the designation KP.
You are in this book
Boundless Chemistry
by Boundless