Concept
Version 15
Created by Boundless
Comparison between Covalent and Ionic Compounds
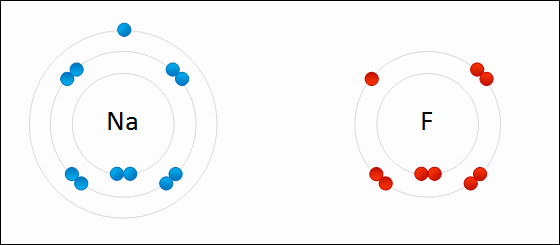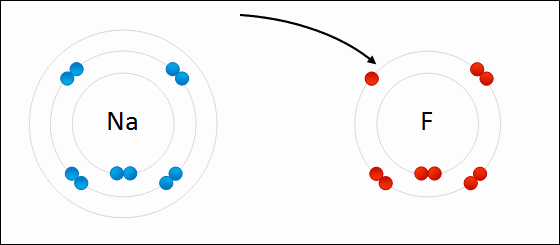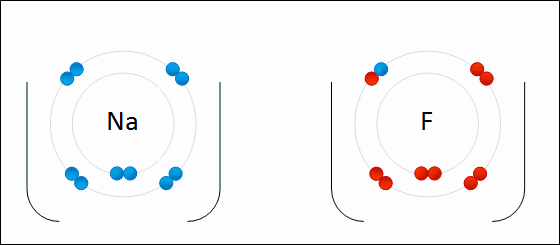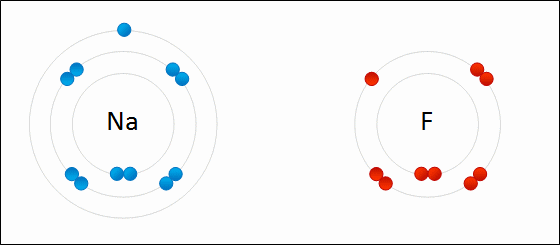
Formation of sodium fluoride (NaF)
The transfer of an electron from a neutral sodium atom to a neutral fluorine atom creates two oppositely charge ions: Na+ and F-. Attraction of the oppositely charged ions is the ionic bond between Na and F.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: