Section 2
The Covalent Bond
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
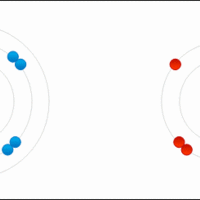
Comparison between Covalent and Ionic Compounds
Covalent and ionic compounds have distinct physical properties.
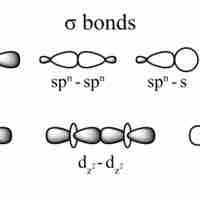
Single Covalent Bonds
Single covalent bonds are sigma bonds, which occur when one pair of electrons is shared between atoms.
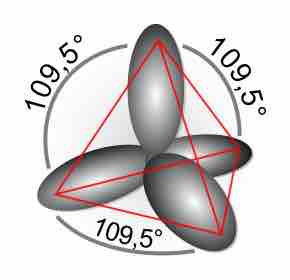
Double and Triple Covalent Bonds
Double and triple bonds, comprised of sigma and pi bonds, increase the stability and restrict the geometry of a compound.
Physical Properties of Covalent Molecules
The covalent bonding model helps predict many of the physical properties of compounds.