Concept
Version 9
Created by Boundless
Hybridization in Molecules Containing Double and Triple Bonds
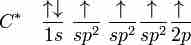
sp2 hybridization
In ethene, carbon sp2 hybridizes, because one π (pi) bond is required for the double bond between the carbons, and only three σ bonds form per carbon atom.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: