Section 4
Valence Bond Theory
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
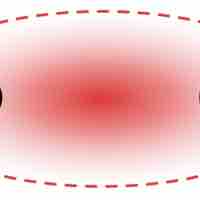
Explanation of Valence Bond Theory
Valence bond theory states that overlap between two atomic orbitals forms a covalent bond between two atoms.
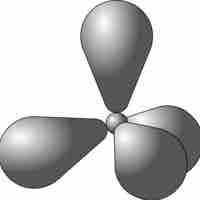
sp3 Hybridization
sp3 hybrid orbitals form when a single s and three p orbitals hybridize.
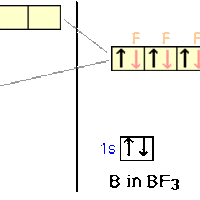
sp2 Hybridization
sp2 hybridization occurs between one s-orbital and two p-orbitals.
sp Hybridization
sp hybrid orbitals form from one s-orbital and one p-orbital.
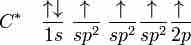
Hybridization in Molecules Containing Double and Triple Bonds
sp2, sp hybridizations, and pi-bonding can be used to describe the chemical bonding in molecules with double and triple bonds.