Section 2
Potential, Kinetic, Free, and Activation Energy
Book
Version 32
By Boundless
By Boundless
Boundless Biology
Biology
by Boundless
4 concepts
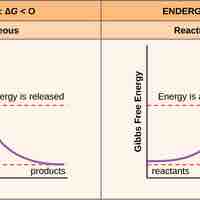
Free Energy
Free energy, called Gibbs free energy (G), is usable energy or energy that is available to do work.

The First Law of Thermodynamics
The first law of thermodynamics states that energy can be transferred or transformed, but cannot be created or destroyed.
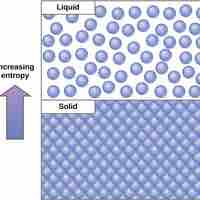
The Second Law of Thermodynamics
The second law of thermodynamics states that every energy transfer increases the entropy of the universe due to the loss of usable energy.
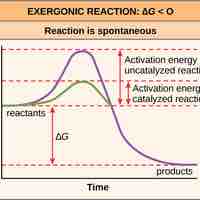
Activation Energy
Activation energy is the energy required for a reaction to occur, and determines its rate.