Section 8
Gas Laws
Book
Version 29
By Boundless
By Boundless
Boundless Anatomy and Physiology
Physiology
by Boundless
2 concepts
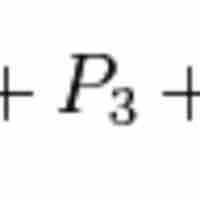
Dalton's Law of Partial Pressure
Dalton's law of partial pressures states that the pressure of a mixture of gases is the sum of the pressures of the individual components.
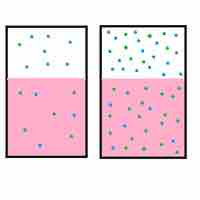
Henry's Law
Henry's law states that the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas.