Section 2
The First Law of Thermodynamics
Book
Version 3
By Boundless
By Boundless
Boundless Physics
Physics
by Boundless
5 concepts
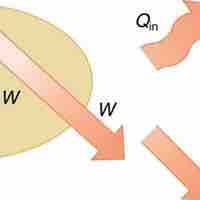
The First Law
The 1st law of thermodynamics states that internal energy change of a system equals net heat transfer minus net work done by the system.
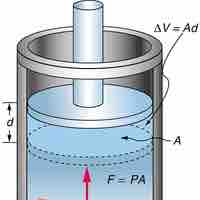
Constant Pressure and Volume
Isobaric process is one in which a gas does work at constant pressure, while an isochoric process is one in which volume is kept constant.
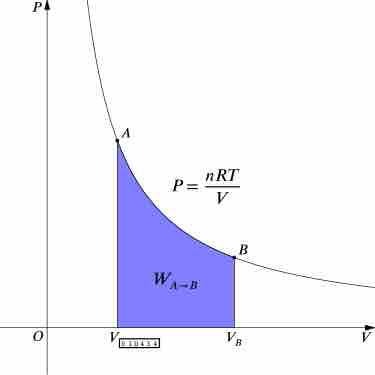
Isothermal Processes
An isothermal process is a change of a thermodynamic system, in which the temperature remains constant.
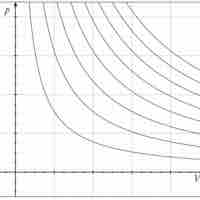
Adiabatic Processes
An adiabatic process is any process occurring without gain or loss of heat within a system.
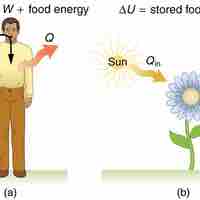
Human Metabolism
The 1st law of thermodynamics explains human metabolism: the conversion of food into energy that is used by the body to perform activities.