Section 3
Coordination Compounds
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
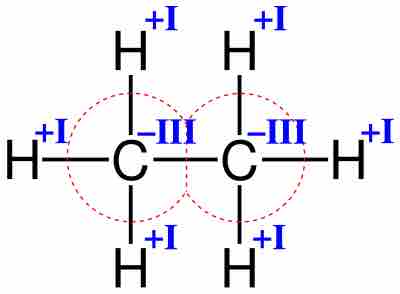
Oxidation Numbers of Metals in Coordination Compounds
Transition metals typically form several oxidation states and therefore have several oxidation numbers.
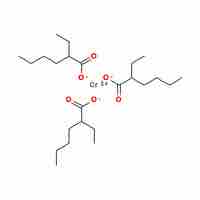
Naming Coordination Compounds
Transition-metal and coordination compounds are named using a set of rules that describe oxidation numbers and anion and cation composition.
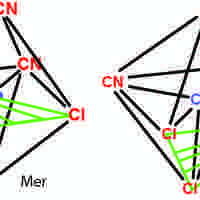
Isomers in Coordination Compounds
Coordination stereoisomers have the same bonds in different orientations; structural isomers have different bonding orientations.

Coordination Number, Ligands, and Geometries
The coordination number determines the number of ligands attached to the central ion and the overall shape of the complex.