Section 5
Bonding in Coordination Compounds: Crystal Field Theory
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
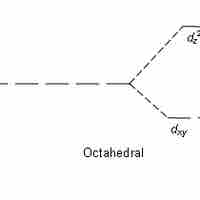
Crystal Field Theory
Crystal field theory states that d or f orbital degeneracy can be broken by the electric field produced by ligands, stabilizing the complex.
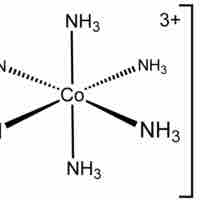
Octahedral Complexes
Octahedral complexes have six ligands symmetrically arranged around a central atom, defining the vertices of an octahedron.
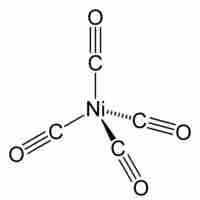
Tetrahedral and Square Planar Complexes
Both tetrahedral and square planar complexes have a central atom with four substituents.

Color
Transition metal complexes are often colored due to either d-d or change band electron transitions induced by the absorption of light.
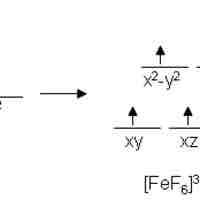
Magnetic Properties
Metal complexes that have unpaired electrons are magnetic.