Section 3
Gibbs Free Energy
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
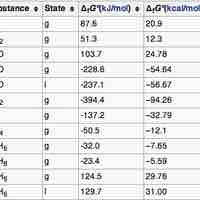
Standard Free Energy Changes
The standard Gibbs Free Energy is calculated using the free energy of formation of each component of a reaction at standard pressure.
Free Energy Changes in Chemical Reactions
ΔG determines the direction and extent of chemical change.
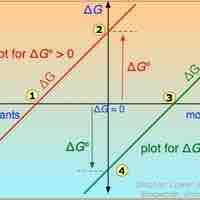
Free Energy Changes for Nonstandard States
A single reaction can have an infinite number of ΔG values, reflecting the infinite possible compositions between reactants and products.

Pressure and Free Energy
Gibbs free energy is dependent on pressure.
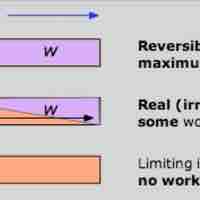
Free Energy and Work
The Gibbs free energy is the maximum amount of non-expansion work that can be extracted from a closed system.