Section 5
Standard Enthalpy of Formation and Reaction
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts

Standard States and Standard Enthalpy Changes
The standard enthalpy of formation refers to the enthalpy change when one mole of a compound is formed from its elements.
Standard Enthalpy of Reaction
The standard enthalpy of reaction is the enthalpy change that occurs in a system when a chemical reaction transforms one mole of matter under standard conditions.
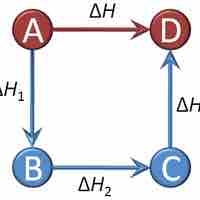
Hess's Law
Hess's Law sums the changes in enthalpy for a series of intermediate reaction steps to find the overall change in enthalpy for a reaction.
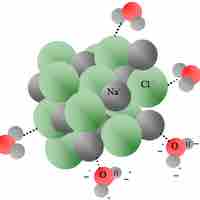
Heat of Solution
Heat of solution refers to the change in enthalpy when a solute is dissolved into a solvent.