Section 4
Calorimetry
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
Specific Heat and Heat Capacity
Heat capacity is a measure of the amount of heat energy required to change the temperature of a pure substance by a given amount.
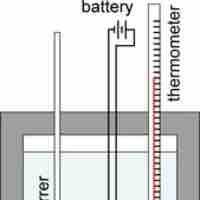
Constant-Volume Calorimetry
Constant-volume calorimeters, such as bomb calorimeters, are used to measure the heat of combustion of a reaction.
Constant-Pressure Calorimetry
A constant-pressure calorimeter measures the change in enthalpy of a reaction at constant pressure.