Section 4
Colligative Properties of Nonelectrolyte Solutions
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts

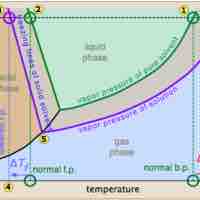
Vapor Pressure of Nonelectrolyte Solutions
The vapor pressure of a solution is directly influenced by the number of solute molecules present in a given amount of solvent.
Freezing Point Depression
Freezing point depression is a colligative property observed in solutions, brought on by the introduction of solute molecules to a solvent.
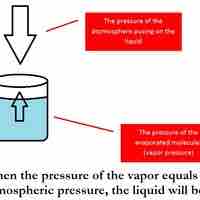
Boiling Point Elevation
The boiling point of a solvent is elevated in the presence of solutes.
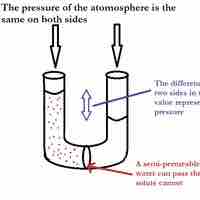
Osmotic Pressure
Osmotic pressure is the pressure needed to nullify the effects of osmosis and is directly influenced by the amount of solute in the system.