Section 1
The History of the Periodic Table
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
6 concepts
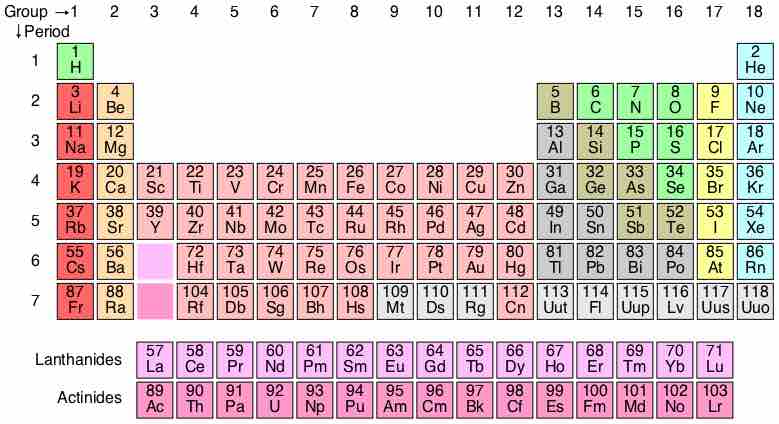
Development of the Periodic Table
The periodic table is a methodical arrangement of the chemical elements, organized on the basis of their electron configurations.

Periods 1 through 3
Elements of the same period have the same number of electron shells.

Transition Metals
The d-block elements are commonly known as transition metals or transition elements.
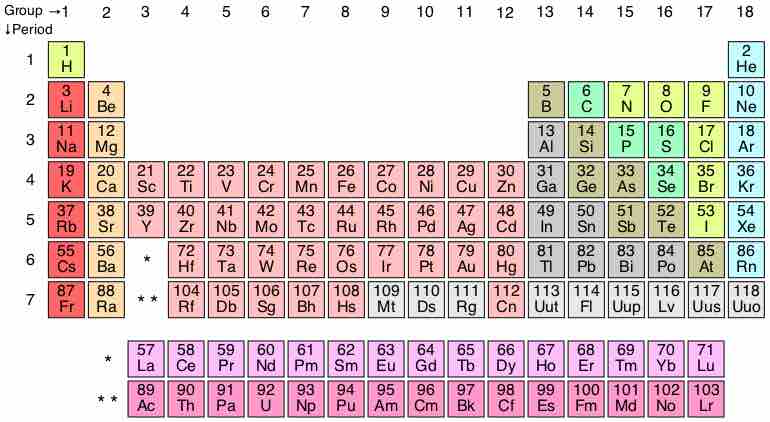
The Bottom of the Periodic Table
The periodic table currently contains 7 periods, but theorists predict that two additional periods may exist.
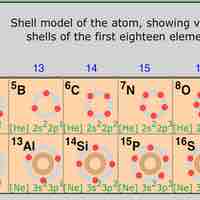
Periodic Table Position and Electron Configuration
The position of elements on the periodic table is directly related to their electron configurations.

Electron Configuration of Cations and Anions
The elements on the periodic table exhibit different levels of reactivity based on the number of electrons in their highest energy shells.