Section 8
Phase Changes
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
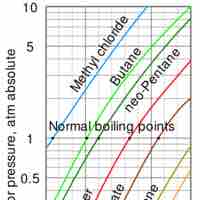
Liquid to Gas Phase Transition
Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase.
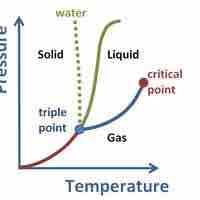
Supercritical Fluids
A supercritical fluid is a substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist.

Liquid to Solid Phase Transition
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered to its freezing point.

Solid to Gas Phase Transition
Sublimation is the phase transition from the solid to the gaseous phase, without passing through an intermediate liquid phase.
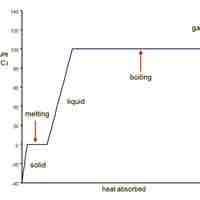
Heating Curve for Water
Water transitions from ice to liquid to water vapor as heat is added to it.