Section 6
Kinetic Molecular Theory
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
Kinetic Molecular Theory and Gas Laws
Kinetic Molecular Theory explains the macroscopic properties of gases and can be used to understand and explain the gas laws.
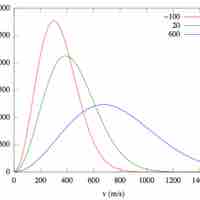
Distribution of Molecular Speeds and Collision Frequency
The Maxwell-Boltzmann Distribution describes the average molecular speeds for a collection of gas particles at a given temperature.
Root-Mean-Square Speed
The root-mean-square speed measures the average speed of particles in a gas, defined as
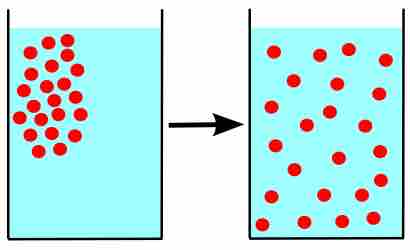
Gas Diffusion and Effusion
Due to their constant, random motion, gas molecules diffuse into areas of lower concentration, and effuse through tiny openings.