Section 2
Gas Laws
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
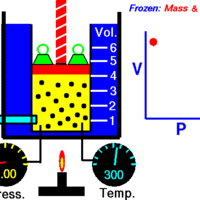
Boyle's Law: Volume and Pressure
Boyle's Law describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature.
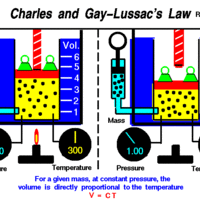
Charles' and Gay-Lussac's Law: Temperature and Volume
Charles' and Gay-Lussac's Law states that at constant pressure, temperature and volume are directly proportional.
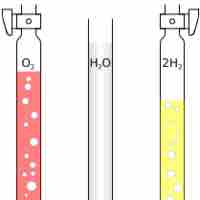
Avogadro's Law: Volume and Amount
Avogadro's Law states that at the same temperature and pressure, equal volumes of different gases contain an equal number of particles.