Concept
Version 8
Created by Boundless
Predicting Spontaneous Direction of a Redox Reaction
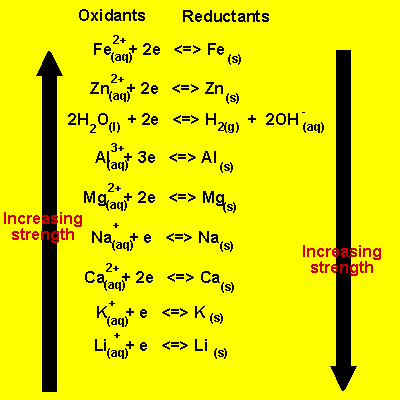
Electrochemical series
In order to predict if two reactants will take part in a spontaneous redox reaction, it is important to know how they rank in an electrochemical series.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"redox reactions - predicting spontaneous redox reactions."
http://www.dynamicscience.com.au/tester/solutions/chemistry/redox/oxidisinreducinstrengthspontaneous.htm
Dynamic Science
CC BY-SA.