Section 3
Lewis Dot Symbols and Lewis Structures
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
Representing Valence Electrons in Lewis Symbols
Lewis symbols use dots to visually represent the valence electrons of an atom.
Writing Lewis Symbols for Atoms
The Lewis symbol for an atom depicts its valence electrons as dots around the symbol for the element.
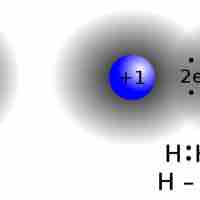
Introduction to Lewis Structures for Covalent Molecules
In covalent molecules, atoms share pairs of electrons in order to achieve a full valence level.
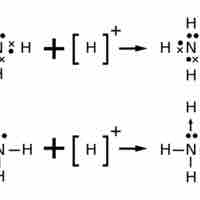
Lewis Structures for Polyatomic Ions
The Lewis structure of an ion is placed in brackets and its charge is written as a superscript outside of the brackets, on the upper right.