Section 6
Exceptions to the Octet Rule
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
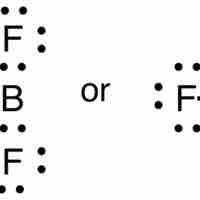
The Incomplete Octet
While most elements below atomic number 20 follow the octet rule, several exceptions exist, including compounds of boron and aluminum.
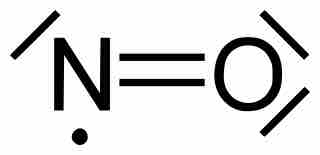
Odd-Electron Molecules
Molecules with an odd number of electrons disobey the octet rule.
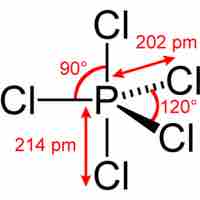
The Expanded Octet
Main group elements in the third period and below form compounds that deviate from the octet rule by having more than 8 valence electrons.