Section 7
Bond Energy and Enthalpy
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
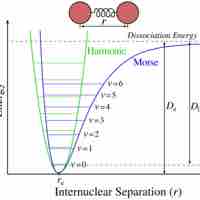
Bond Energy
Bond energy is the measure of bond strength. In order to turn one mole of a molecule into its constituent atoms, an amount of heat equal to the bond energy needs to be put into the system.
.jpg)
Bond Enthalpy
Bond enthalpy is defined as the enthalpy change when a covalent bond is cleaved by homolysis.
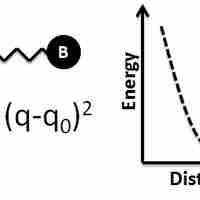
Bond Lengths
Bond length between two atoms depends on factors such as the orbital hybridization and the electronic nature of the components.