Section 7
Naming Compounds
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
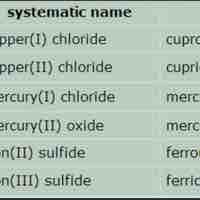
Naming Ionic Compounds
An ionic compound is named first by its cation and then by its anion.
Naming Molecular Compounds
Molecular compounds are named using a systematic approach of prefixes to indicate the number of each element present in the compound.
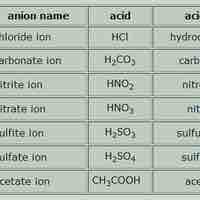
Naming Acids and Bases
Acid names are based on the anion they form when dissolved in water; base names follow the rules for ionic, organic, or molecular compounds.

Naming Hydrates
The name of a hydrate follows a set pattern: the name of the ionic compound followed by a numerical prefix and the suffix -hydrate.
Naming Familiar Inorganic Compounds
Familiar inorganic and organic compounds are often known by their common, or "trivial," names.