Section 1
History of Atomic Structure
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
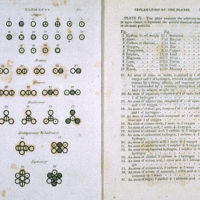
Early Ideas about Atoms
The concept of the atom as an indivisible building block of matter was recorded as early as the 5th century BCE.

The Law of Conservation of Mass
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed.

The Law of Definite Composition
The law of definite composition states that chemical compounds are composed of a fixed ratio of elements as determined by mass.
The Law of Multiple Proportions
The law of multiple proportions states that elements combine in small whole number ratios to form compounds.
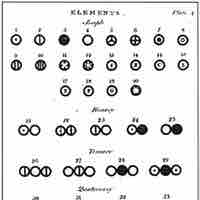
John Dalton and Atomic Theory
Dalton introduced a theory that proposed that elements differed due to the mass of their atoms.