Section 2
Precipitation Reactions
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts

Precipitation Reactions
Precipitation reactions transform ions into an insoluble salt in aqueous solution.
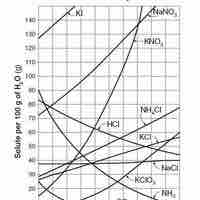
Solubility
Solubility is the relative ability of a solute (solid, liquid, or gas) to dissolve into a solvent and form a solution.

Molecular, Ionic, and Complete Ionic Equations
Precipitation reactions can be written as molecular, ionic, or complete ionic equations.