Section 4
Oxidation-Reduction Reactions
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts

Oxidation States
Oxidation state is the hypothetical charge of an atom if all of its bonds to other atoms were completely ionic.
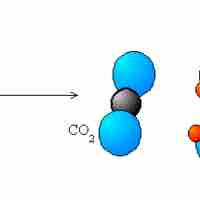
Types of Redox Reactions
The five main types of redox reactions are combination, decomposition, displacement, combustion, and disproportionation.
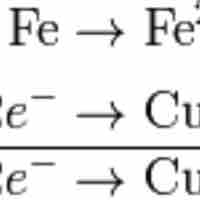
Balancing Redox Equations
Balancing redox reactions involves splitting the reaction into two half-reactions.
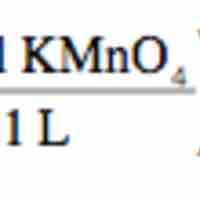
Redox Titrations
Redox titration determines the concentration of an analyte containing either an oxidizing or a reducing agent.