Section 3
Molecular Shape and Polarity
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
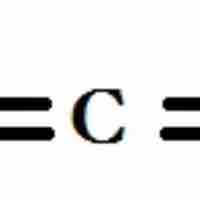
Dipole Moment
A dipole exists when a molecule has areas of asymmetrical positive and negative charge.
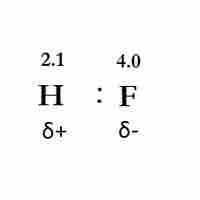
Bond Polarity
Bond polarity exists when two bonded atoms unequally share electrons, resulting in a negative and a positive end.
Percent Ionic Character and Bond Angle
Chemical bonds are more varied than terminology might suggest; they exist on a spectrum between purely ionic and purely covalent bonds.