Section 5
Molecular Orbital Theory
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
6 concepts
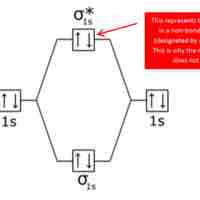
Bonding and Antibonding Molecular Orbitals
Bonding and antibonding orbitals are illustrated in MO diagrams, and are useful for predicting the strength and existence of chemical bonds.
Bond Order
Bond order is the number of chemical bonds between a pair of atoms.
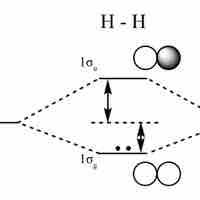
Linear Combination of Atomic Orbitals (LCAO)
An LCAO approximation is a quantum superposition of atomic orbitals, used to calculate molecular orbitals in quantum chemistry.

Homonuclear Diatomic Molecules
Homonuclear diatomic molecules are composed of only one element.
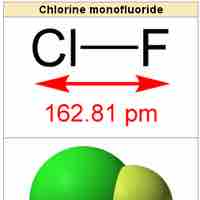
Heteronuclear Diatomic Molecules
Heteronuclear diatomic molecules are composed of two atoms of two different elements.
Polyatomic Molecules
A polyatomic molecule is a single entity composed of at least three covalently-bonded atoms.