Section 2
Molecular Geometry
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
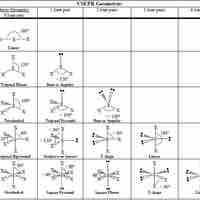
Table of Geometries
The VSPER theory detremines molecular geometries (linear, trigonal, trigonal bipyramidal, tetrahedral, and octahedral).
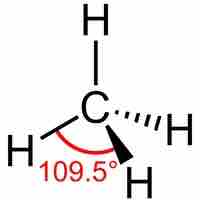
Molecular Geometries
The VSEPR theory describes five main shapes of simple molecules: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
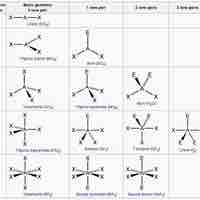
Lone Electron Pairs
Nonbonding electrons are in orbitals that occupy space, repel the other orbitals, and change a molecule's shape.