Section 3
Strength of Bases
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts

Strong Bases
Strong bases either dissociate completely in solution to yield hydroxide ions, or deprotonate water to yield hydroxide ions.
Weak Bases
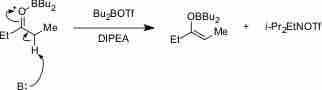
In aqueous solution, a weak base reacts incompletely with water to yield hydroxide ions.
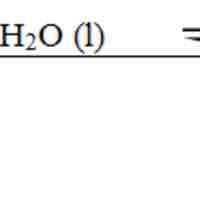
Base Dissociation Constant
The base dissociation constant (Kb) measures a base's relative strength.