Section 6
Acid-Base Properties of Salts
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
.jpg)
Salts that Produce Basic Solutions
When dissolved in water, a basic salt yields a solution with pH greater than 7.0.
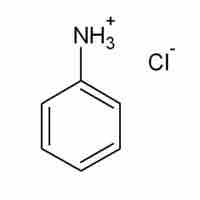
Salts that Produce Acidic Solutions
When dissolved in water, acidic salts form solutions with pH less than 7.0.
Overview of the Acid-Base Properties of Salt
Some salts, such as ammonium bicarbonate (NH4HCO3), contain cations and anions that can both undergo hydrolysis.