Concept
Version 8
Created by Boundless
Basic and Amphoteric Hydroxides
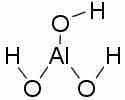
Aluminum hydroxide
Aluminum hydroxide can act as either a Bronsted-Lowry base, by accepting protons from an acidic solution, or as a Lewis acid, by accepting an electron pair from hydroxide ions in a basic solution.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Aluminium hydroxide."
http://commons.wikimedia.org/wiki/File:Aluminium_hydroxide.JPG
Wikimedia
CC BY-SA.