Section 2
Water
By Boundless

Water's polarity is responsible for many of its properties including its attractiveness to other molecules.
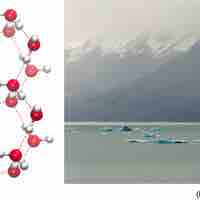
The orientation of hydrogen bonds as water changes states dictates the properties of water in its gaseous, liquid, and solid forms.
Water is able to absorb a high amount of heat before increasing in temperature, allowing humans to maintain body temperature.
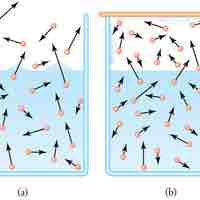
Evaporation of water requires a substantial amount of energy due to the high heat of vaporization of water.
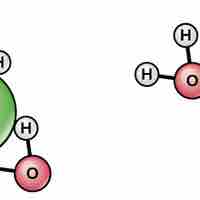
Water's polarity makes it an excellent solvent for other polar molecules and ions.

Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules.
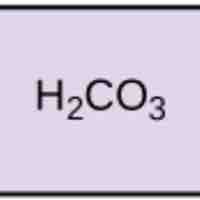
Acids dissociate into H+ and lower pH, while bases dissociate into OH- and raise pH; buffers can absorb these excess ions to maintain pH.