Concept
Version 9
Created by Boundless
Electron Shells and the Bohr Model
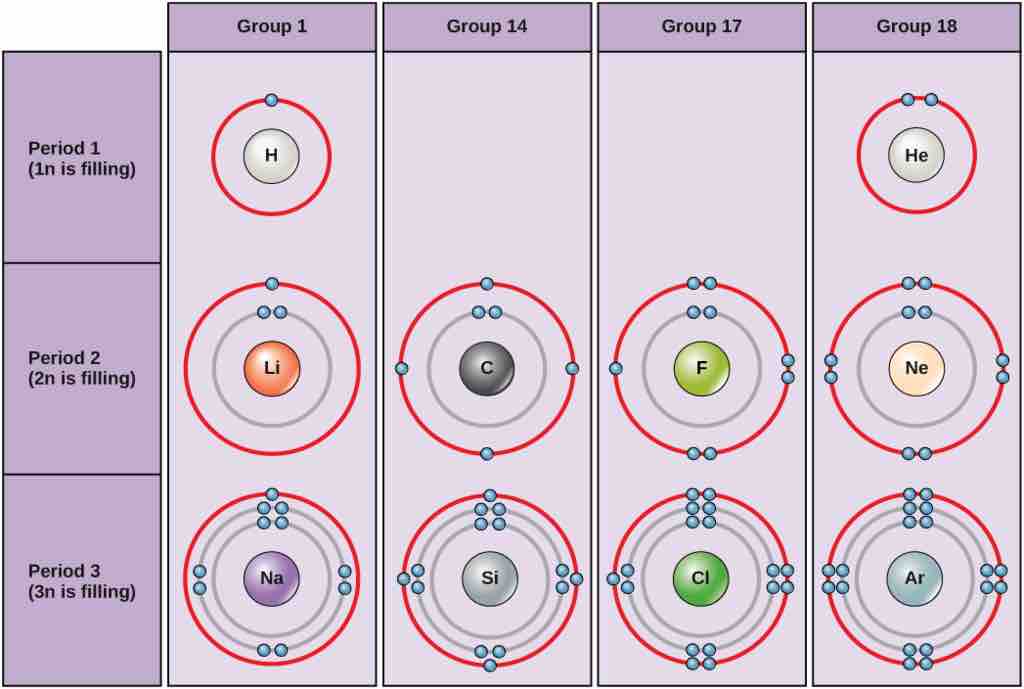
Bohr diagrams
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron configuration.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Atoms, Isotopes, Ions, and Molecules: The Building Blocks. October 16, 2013."
http://cnx.org/content/m44390/latest/Figure_02_01_06.png
OpenStax CNX
CC BY 3.0.